Should You Trust In-House Supplement Testing? A Deep Look at Internal Quality Control
Abstract / Summary
In-house supplement testing plays a vital role in quality control and cGMP compliance, but it is not a substitute for independent lab verification. This article explores the functions, strengths, and limitations of internal testing conducted by supplement brands, highlighting how it contributes to manufacturing integrity while falling short in areas like transparency, contaminant screening, and consumer trust. By comparing in-house methods to ISO-accredited third-party laboratories, we uncover the critical importance of scientific neutrality and objective testing protocols. From raw material verification to real-world product validation, the future of supplement credibility depends on a layered approach that combines internal discipline with external accountability.
By Sighed Effects — May 4, 2025

Table of Contents
- What Is In-House Testing?
- Why Do Brands Use In-House Testing?
- What In-House Labs Typically Test For
- Limitations and Blind Spots of In-House Testing
- Regulatory Standards and Legal Role of In-House Testing
- When In-House Testing Is Trustworthy (and When It’s Not)
- How Brands Present In-House Testing in Marketing
- When In-House Testing Falls Short
- The Role of In-House Labs in cGMP Compliance
- Why Independent Verification Still Matters
What Is In-House Testing?
In-house testing refers to quality control procedures performed by the supplement manufacturer itself—either on-site at its own facility or through a contracted lab that is financially affiliated with the brand. These tests are typically carried out during production to assess ingredient identity, potency, safety, and compliance with manufacturing specifications.
Unlike independent lab testing, which is performed by third parties with no financial stake in the product, in-house testing operates within the supply chain. This allows companies to test materials at multiple stages: when raw ingredients arrive, during formulation, and as part of final product release. In theory, this continuous oversight should improve product quality. But in practice, in-house testing raises questions about transparency, accountability, and the potential for bias.
For regulatory purposes, in-house testing is often sufficient to meet current good manufacturing practices (cGMPs) required by the U.S. Food and Drug Administration (FDA). However, it does not constitute third-party verification. Consumers frequently see “lab tested” on supplement labels without knowing whether the testing was done internally or independently—a distinction that can drastically affect credibility.
As supplement quality becomes a major concern for health-conscious consumers, understanding the scope and limitations of in-house testing is critical. This article explores how in-house testing works, what it can and cannot detect, how it compares to third-party analysis, and how to interpret testing claims made by supplement brands.
Why Do Brands Use In-House Testing?
In-house testing is not inherently suspicious or deceptive. For many supplement companies—especially those with large-scale operations or vertically integrated supply chains—it’s a practical necessity. Internal quality control labs allow brands to rapidly assess raw materials, monitor production consistency, and ensure that each batch meets internal specifications before being released to market.
Manufacturers often use in-house testing to comply with current Good Manufacturing Practices (cGMPs), as required by the U.S. FDA and equivalent agencies abroad. These regulations mandate that companies validate the identity, purity, strength, and composition of their ingredients. While in-house labs may lack the independence of third-party testing, they can still meet legal standards—provided the equipment is calibrated, the technicians are trained, and documentation is properly maintained.
In-house testing is also cost-effective. Sending every batch to a third-party lab for analysis can be expensive and time-consuming, especially for high-volume products. By maintaining internal control over quality checks, companies reduce turnaround times and avoid costly disruptions to their manufacturing schedule.
Some brands take in-house testing a step further by building full-service analytical labs equipped with high-performance instruments like HPLC (high-performance liquid chromatography), ICP-MS (inductively coupled plasma mass spectrometry), and microbial plating systems. These companies often emphasize their quality infrastructure in marketing materials, highlighting their investment in science and self-regulation.
In summary, brands rely on in-house testing for speed, regulatory compliance, operational efficiency, and cost control. The presence of internal labs is not a red flag—but the lack of external validation can become one, especially when brands make sweeping purity or potency claims without publishing third-party data.
What In-House Labs Typically Test For
In-house testing generally focuses on quality control parameters that are most relevant during production. These include verifying the identity of raw materials, assessing ingredient potency, checking for microbial contamination, and ensuring the final product meets visual and physical specifications such as color, texture, and capsule integrity. While these tests are essential for ensuring batch consistency, they represent only a subset of what a comprehensive independent lab analysis might include.
Most in-house labs perform identity verification using simple analytical techniques such as FTIR (Fourier-transform infrared spectroscopy) or colorimetric testing. These methods can confirm whether a raw material matches a known standard, but they may not detect adulteration, low-grade variants, or substituted compounds unless the deviations are obvious.
For potency testing, manufacturers typically assess the concentration of active ingredients such as vitamins, minerals, or herbal extracts. This is often done using HPLC or UV-visible spectrophotometry. These measurements help ensure that each batch meets its declared dosage—but again, results may not be published, and the calibration of in-house equipment is not always independently verified.
Microbial testing is another common focus. Brands check for the presence of harmful bacteria (such as Salmonella or E. coli) and total plate counts for yeast and mold. These tests are necessary for safety, especially in probiotics, herbal supplements, or powders that are prone to moisture exposure. Some companies use PCR-based methods or third-party culture tests for confirmation, while others rely on internal plating with variable reliability.
Additional assessments may include capsule disintegration tests, tablet hardness testing, and pH analysis—especially for supplements intended to release in specific parts of the gastrointestinal tract. However, few in-house labs test for contaminants like heavy metals, pesticides, or residual solvents unless the brand has invested in advanced equipment and personnel.
The limitation is clear: in-house labs are typically optimized for speed and operational control, not comprehensive third-party validation. What they test for is often what’s easiest to test for—leaving gaps in contamination screening, ingredient substitution detection, and overall label accuracy.

Limitations and Blind Spots of In-House Testing
While in-house testing fulfills important quality control roles, it has well-documented limitations. Internal labs are not typically structured to perform the full range of contaminant screening, identity authentication, or long-term stability analysis that independent third-party labs provide. These gaps can lead to undetected risks, mislabeled products, and misleading claims.
One major limitation is scope of testing. Internal labs tend to focus on a handful of ingredients per batch—typically the active components that define the supplement’s market appeal. In contrast, third-party labs often run broader panels, including screening for heavy metals, microbial load, pesticide residues, residual solvents, undeclared pharmaceuticals, and allergens.
Another blind spot is formulation complexity. When products contain dozens of ingredients—especially in proprietary blends—it becomes difficult for in-house teams to verify each one. Without robust testing methods and reference standards for every compound, companies may miss ingredient substitutions, potency degradation, or improper excipient levels that affect bioavailability.
There’s also the issue of batch-to-batch variation. In-house testing is typically performed on representative samples, not every unit. This leaves room for inconsistency, especially in products with natural variability (e.g., herbal extracts). If the sampling process isn’t statistically rigorous, entire batches could deviate from label claims without detection.
In many cases, in-house testing lacks method validation and accreditation. While third-party labs must adhere to ISO/IEC 17025 or equivalent standards, internal labs may use unvalidated methods, improperly calibrated equipment, or underqualified staff. Without documentation of precision, accuracy, and reproducibility, test results can’t be confidently compared across labs or brands.
Perhaps the most critical blind spot is conflict of interest. When a company tests its own product, there is an implicit incentive to report favorable outcomes. Even with the best intentions, confirmation bias, selective data presentation, and omission of failed results can compromise transparency. Without external oversight, consumers have no way to verify whether the reported data reflects the actual product in their hands.
These limitations do not mean in-house testing is worthless—but they underscore the need for independent verification. As supplements play increasingly significant roles in healthcare, consumers and clinicians need more than internal assurance. They need external accountability.

Regulatory Standards and Legal Role of In-House Testing
In-house testing is not merely a matter of operational preference—it plays a key role in meeting legal obligations under supplement manufacturing regulations. In the United States, the Food and Drug Administration (FDA) requires dietary supplement manufacturers to comply with current Good Manufacturing Practices (cGMPs), which include specific guidelines for quality control testing conducted by the manufacturer itself.
According to 21 CFR Part 111, manufacturers must verify the identity of each dietary ingredient and ensure that the supplement meets established specifications for strength, composition, and purity. This often requires in-house analytical procedures such as identity testing for raw materials and batch-level confirmation of active ingredient concentrations. Brands are also expected to test for contamination, especially microbial growth and physical impurities like metal shavings or foreign matter.
While these regulations require documented testing procedures, they do not dictate the use of third-party labs. Manufacturers are allowed to conduct all required testing internally—as long as the methods are scientifically valid and documented. This leaves room for interpretation, and the quality of testing varies widely between companies.
To support these requirements, the FDA expects companies to maintain Standard Operating Procedures (SOPs), retain detailed test records, and ensure that laboratory instruments are calibrated and validated. During inspections, FDA auditors may review in-house lab documentation, interview staff, and examine testing logs. Failure to comply can result in warning letters, product recalls, or injunctions—but proactive FDA inspections are relatively rare.
Globally, similar standards exist. The European Union follows its own version of GMP compliance through the European Medicines Agency (EMA) and national health authorities. In Canada, the Natural and Non-Prescription Health Products Directorate (NNHPD) requires quality testing aligned with Health Canada regulations. However, as in the U.S., independent verification is not universally mandated—making in-house testing the default baseline.
In practice, regulators assume that brands are acting in good faith. The system relies on accurate record-keeping, internal controls, and post-market surveillance. But without public-facing documentation—such as batch-specific Certificates of Analysis from independent labs—consumers have no direct way to verify compliance. What’s legal isn’t always what’s transparent.
In-house testing may satisfy regulatory minimums, but it is not the gold standard. It is up to brands to go beyond compliance and prove their commitment to transparency—and up to consumers to demand more than the bare legal threshold.

When In-House Testing Is Trustworthy (and When It’s Not)
In-house testing can be highly reliable—under the right conditions. The key determinant is not whether testing is internal, but how it is conducted, documented, and disclosed. Brands that invest in high-quality lab infrastructure, hire credentialed scientists, and follow rigorous testing protocols may produce results as accurate as those from independent labs. But these brands are the exception, not the norm.
Trustworthy in-house testing is often found in companies that:
- Use validated analytical methods (e.g., USP, AOAC)
- Calibrate instruments regularly and maintain traceable records
- Document standard operating procedures (SOPs) and batch testing logs
- Employ qualified personnel, such as PhD chemists or trained QC technicians
- Subject internal labs to external audits or proficiency testing
These practices show a commitment to scientific integrity—even if the lab is owned by the brand. Some premium supplement manufacturers maintain pharmaceutical-grade labs and routinely pass third-party audits by the FDA, Health Canada, or NSF.
However, in-house testing becomes less trustworthy when:
- Testing is limited to raw materials and not the finished product
- Results are not batch-specific or traceable to lot numbers
- The brand refuses to disclose testing data to consumers
- Certificates of Analysis (COAs) are vague, undated, or lack a lab name
- Lab documentation is inconsistent, self-signed, or missing critical data
Brands may claim to follow internal quality standards without showing proof. When a company makes generalized statements like “every batch is tested” but offers no test results, it’s reasonable to question the depth and validity of those claims. In such cases, independent lab data becomes a litmus test for consumer trust.
Ultimately, transparency is the dividing line. A brand with strong in-house testing should be willing to disclose its protocols, instrumentation, and quality metrics. If that information is unavailable, incomplete, or inconsistent, the consumer has every right to look elsewhere.

How Brands Present In-House Testing in Marketing
Supplement companies frequently reference in-house testing in their marketing—but the way this information is presented can range from honest to misleading. While some brands use in-house data to reinforce trust and transparency, others use vague or deceptive language to create the illusion of third-party validation.
A common phrase is “every batch is tested,” which sounds reassuring but often lacks specifics. What kind of testing was performed? Was the final product tested, or just raw materials? Was testing performed on every batch or just representative samples? Without context, this type of claim is little more than a marketing slogan.
Other brands showcase Certificates of Analysis (COAs) that are internally generated. These documents may include the brand’s own logo and test results, but omit the name of the lab or details about the methodology. In some cases, COAs are stylized to appear like third-party reports even though the data originates entirely from the manufacturer’s own facilities.
Some brands attempt to bridge the trust gap by saying their internal labs are “FDA compliant” or “meet pharmaceutical standards.” While these phrases suggest regulatory credibility, they are not equivalent to independent verification. The FDA does not certify or approve labs, and “pharmaceutical-grade” has no legal definition in the supplement space.
Visual design can also play a role in how in-house testing is marketed. Brands may display imagery of lab technicians in white coats, analytical instruments, or scientific charts to create an aura of legitimacy. But unless the data itself is traceable, specific, and reproducible, such imagery is purely aesthetic.
To responsibly present in-house testing, a brand should:
- Clearly state what was tested, how often, and using which methods
- Specify whether testing was internal or independent
- Provide access to batch-specific COAs linked to lot numbers
- Disclose lab qualifications, accreditations, and testing protocols
- Use plain language—not just legal or scientific jargon—to explain results
When used properly, in-house testing claims can enhance brand credibility. But when brands overstate or obscure the nature of their testing, they risk eroding consumer trust—and attracting scrutiny from regulators, watchdogs, or savvy shoppers who know what real transparency looks like.

When In-House Testing Falls Short
Despite its value in internal quality control, in-house testing has significant limitations that consumers and clinicians should be aware of. These limitations are not necessarily due to malice or deception—they often stem from structural, logistical, or economic constraints within the supplement industry. Still, the consequences can be meaningful when it comes to product safety and efficacy.
One of the most common issues is limited testing scope. Many brands focus their in-house efforts on verifying ingredient identity and basic potency, but skip comprehensive testing for contaminants like heavy metals, pesticides, or residual solvents. These omissions may be unintentional or budget-driven, but they leave gaps in the safety profile of the product.
Lack of batch consistency testing is another area of concern. A manufacturer may test one batch of a product and assume subsequent runs are equivalent. However, even small differences in raw materials, manufacturing conditions, or storage environments can alter the supplement’s composition. Without testing every lot—or at least conducting periodic spot checks—there is no assurance that label accuracy is maintained over time.
Another limitation is equipment constraints. Not all in-house labs have access to high-end analytical tools like ICP-MS, GC-MS, or HPLC. Instead, they may rely on lower-sensitivity or semi-quantitative methods, such as thin-layer chromatography (TLC) or basic UV spectrophotometry. These tools may suffice for some tests, but fall short when precise quantification or contamination screening is needed.
Even when adequate equipment is available, operator error or poor training can undermine the reliability of results. Inadequate sample preparation, improper calibration, or flawed documentation can all compromise the outcome. Without external validation or proficiency testing, it’s difficult to know whether in-house results meet scientific standards.
In some cases, data may be withheld or selectively reported. Internal testing that shows negative results—such as ingredient degradation, microbial overgrowth, or heavy metal contamination—may never leave the lab if there is no regulatory obligation to disclose it. In the absence of independent oversight, the public has no way to know what’s being excluded.
Finally, the lack of consumer access to internal testing data remains a persistent weakness. Most brands do not publish in-house COAs, and when they do, the information is often stripped of essential context. This stands in contrast to independent testing, which is typically performed with the intention of public disclosure and consumer accountability.
While in-house testing is better than no testing at all, its limitations should not be overlooked. It offers a baseline level of assurance—but not the depth, neutrality, or credibility that independent verification provides. In high-risk supplements, clinical use cases, or vulnerable populations, third-party testing remains the gold standard.

The Role of In-House Labs in cGMP Compliance
In-house laboratories play a central role in helping supplement manufacturers meet the requirements of Current Good Manufacturing Practices (cGMP), as mandated by the FDA under 21 CFR Part 111. These practices ensure that dietary supplements are produced in a consistent, high-quality manner and are free from contamination or mislabeling. While cGMPs do not require independent testing, they do require robust internal controls—and in-house labs are often the mechanism by which those controls are enforced.
A compliant in-house lab supports multiple stages of production, including:
- Raw material verification – confirming ingredient identity before use
- In-process testing – evaluating product integrity during blending and encapsulation
- Finished product testing – ensuring the final supplement meets label claims and specifications
- Stability and shelf-life testing – assessing how a product degrades over time
- Environmental monitoring – checking the cleanliness and safety of the production environment
These functions help manufacturers prevent cross-contamination, catch formulation errors, and maintain consistent quality across production runs. Labs operating under cGMP guidelines must document their procedures, calibrate their instruments regularly, and maintain auditable records. Failure to follow these standards can result in FDA warning letters, product recalls, or even criminal penalties.
However, cGMP compliance does not mean a company has tested for everything consumers care about. Many cGMP-compliant brands do not test for heavy metals, pesticides, or undeclared drugs unless required by a specific ingredient monograph. Nor do they typically test for bioavailability, disintegration, or oxidation unless the product warrants it.
It’s also important to note that cGMP inspections are infrequent and risk-based. The FDA prioritizes site visits based on factors like complaint history, product type, and past violations. This means that some manufacturers may go years without an inspection—and their in-house lab practices may never be externally reviewed unless a problem arises.
In this context, in-house labs are best understood as tools of internal accountability. They can uphold high manufacturing standards—but only if the brand itself prioritizes accuracy, safety, and documentation. In the absence of independent oversight, the strength of a cGMP-compliant lab ultimately depends on the company’s own integrity.
In the final section, we’ll explore how in-house and independent testing can work together—and why supplement users should be demanding both.
Why Independent Verification Still Matters
Even when a supplement brand has a well-equipped, compliant in-house lab, independent testing remains a critical layer of accountability. It is not a redundancy—it is a safeguard. Independent verification helps close the credibility gap between what a company claims and what a neutral, third-party organization can confirm. For consumers navigating a self-regulated industry, this distinction matters.
Independent labs are not influenced by product sales, branding goals, or internal politics. Their results are often used for public reports, regulatory audits, and scientific publications. The pressure to maintain scientific neutrality creates a higher bar for trust—and provides a check on unintentional errors, biased reporting, or deceptive practices that may occur when testing is handled entirely in-house.
In addition, independent labs often employ more sophisticated and diverse testing equipment than what is feasible in a mid-size brand’s facility. From ICP-MS to GC-MS to DNA authentication platforms, these external labs are built for deep, high-precision analysis. They also typically follow ISO/IEC 17025 accreditation protocols, which mandate internal audits, third-party proficiency testing, and documented procedures for every test performed.
From a consumer perspective, the benefits are clear. Independent lab testing:
- Confirms that in-house data is accurate and unbiased
- Helps detect contaminants that internal labs may miss
- Increases confidence in high-risk categories like nootropics, weight loss, and children’s supplements
- Strengthens brand credibility, especially for practitioner-recommended or clinically used products
- Supports evidence-based decision-making for health professionals and researchers
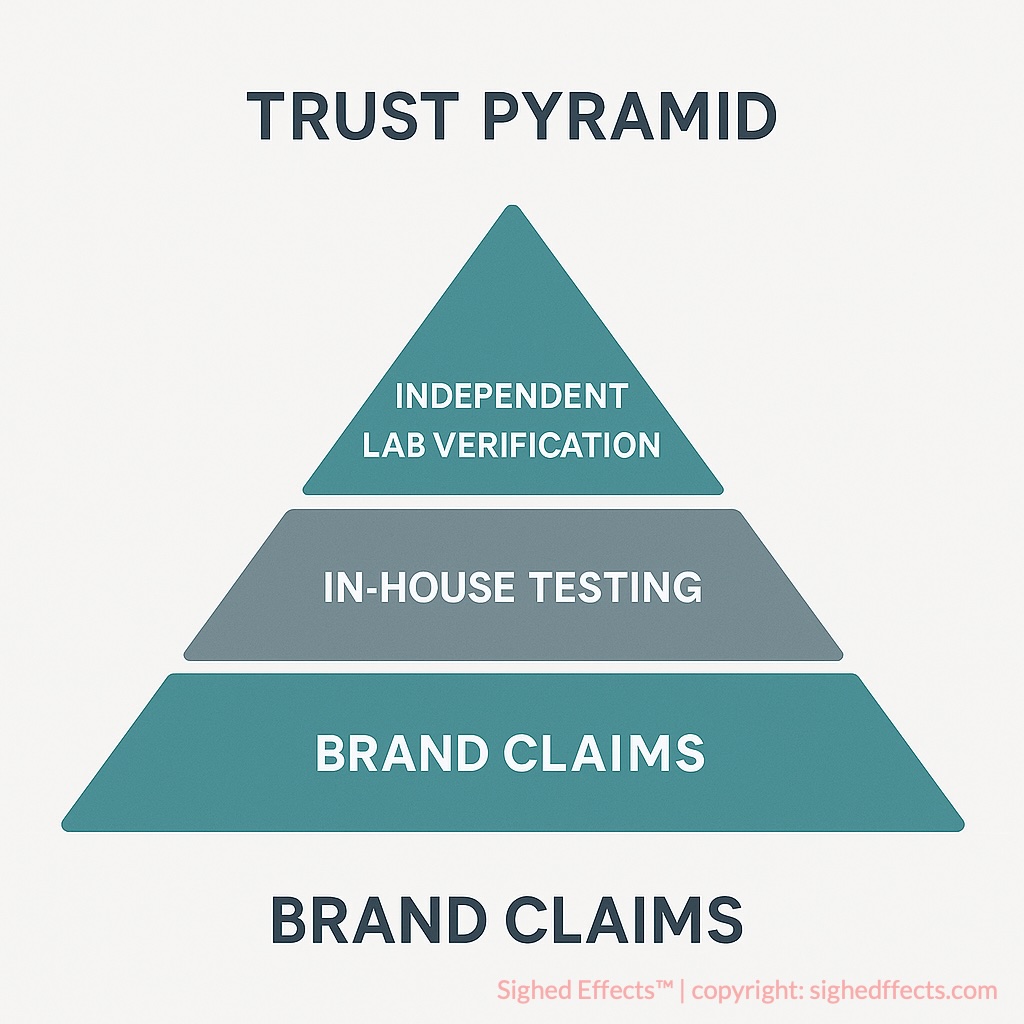
The ideal scenario is not choosing between in-house and independent testing—it’s using both. A strong in-house lab builds day-to-day quality control and supports cGMP compliance. Independent labs add credibility, objectivity, and public accountability. Together, they create a layered system of trust that can withstand scrutiny and serve the end user more effectively.
As supplement science and consumer expectations continue to evolve, so too must the standards for verification. Transparency is no longer a luxury; it’s the baseline. And in this new landscape, independent verification remains the most trusted currency.

Questions or Comments?
If you have a question or comment about this article, feel free to leave it below. All comments are moderated for clarity, accuracy, and relevance.
