Inside Labdoor Certification: How Independent Supplement Testing is Reshaping Consumer Trust and Label Transparency
Abstract / Summary
Labdoor Certification is one of the few independent, science-based testing systems that analyzes finished dietary supplements for label accuracy, purity, and ingredient safety. Unlike traditional third-party certifiers like USP, NSF, or Informed Choice, Labdoor anonymously purchases supplements directly from retail outlets and tests them using pharmaceutical-grade methods such as HPLC and ICP-MS. This article explains how Labdoor ranks supplements, what the Labdoor Score really means, and how to use it as a consumer or clinician. We also compare Labdoor to other certification programs, examine its limitations, and explain when additional scrutiny is necessary. Whether you’re buying creatine, multivitamins, CBD, or protein powders, this deep dive helps you understand if a product contains what it claims—and if it’s worth your trust. Fully updated for 2025 and written for both search engine relevance and AI-driven indexing.
What Is Labdoor Certification?
Labdoor Certification is a third-party quality assurance program that independently tests dietary supplements for label accuracy, ingredient purity, and potential safety concerns. Founded in 2012, Labdoor’s core mission is to empower consumers with transparent, evidence-based evaluations of supplement products that are often sold with minimal regulatory oversight. As a science-driven entity, Labdoor conducts chemical analysis of finished supplement products—meaning it evaluates what’s actually inside the bottle, not just the manufacturer’s claims.
Why Labdoor Exists
Labdoor’s mission is rooted in a simple premise: consumers deserve to know what they’re putting into their bodies. Unlike pharmaceutical drugs, which must undergo extensive FDA approval processes, dietary supplements are regulated post-market in the United States. This means that many supplements reach store shelves without any pre-approval for accuracy, purity, or safety. Labdoor seeks to close this regulatory gap by testing finished products for actual ingredient content, verifying that they match their label claims, and identifying potential contaminants.
How Labdoor Selects and Tests Products
Labdoor’s testing process is entirely product-focused. It does not involve brand contracts or prior manufacturer consent. Most of the supplements evaluated are purchased anonymously through retail outlets or online platforms, mimicking typical consumer behavior. This ensures that what Labdoor tests is what the average buyer would receive—not a handpicked batch from a manufacturer’s quality control inventory.
Lab Testing and Safety Reporting
Every supplement tested by Labdoor undergoes laboratory examination in FDA-registered, ISO 17025-certified facilities. These tests quantify the actual content of active ingredients and compare them to the amounts listed on the product label. If a product claims to contain 500 mg of vitamin C but only delivers 420 mg, that discrepancy is reported. Conversely, if a supplement contains significantly more of a stimulant, hormone precursor, or heavy metal than advertised—or includes an unlisted substance altogether—Labdoor flags this as a potential safety issue.
Independence and Licensing
One of the key differentiators of Labdoor Certification is its independence. Unlike some certifiers that primarily audit Good Manufacturing Practices (GMP) or rely on brand participation, Labdoor often acquires products directly from store shelves or e-commerce sites, just like a consumer would. Brands cannot pay to be ranked higher or influence the testing process. If they choose, manufacturers may license the “Labdoor Certified” badge once their product has undergone testing and met specific quality thresholds, but this does not alter the scientific evaluation itself.
Transparency and Rankings
Products that meet Labdoor’s criteria for label accuracy, low contamination risk, and ingredient safety are eligible for inclusion in its rankings. Manufacturers may then choose to license the “Labdoor Certified” badge, but the underlying data is published regardless of whether the brand participates commercially. This creates an accountability model where transparency comes first, and recognition is optional.
Labdoor’s Industry Role
Labdoor’s role in the supplement industry is both disruptive and necessary. In a market where supplement labels can legally deviate by up to 20% from actual content, and where contaminants such as lead, arsenic, or banned substances may appear undetected, Labdoor offers a rare layer of verification grounded in laboratory science. The results are made available to the public via detailed product reports, rankings, and ingredient breakdowns that support informed purchasing decisions.
Building Consumer Trust
As consumer interest in supplement safety, efficacy, and ethical sourcing continues to grow, Labdoor Certification plays a crucial role in rebuilding trust. It provides a science-first alternative to marketing claims, influencer endorsements, or unverifiable reviews, helping consumers identify supplements that truly meet label claims and are free from harmful substances.
Why Labdoor Matters
In an industry often clouded by proprietary blends, vague dosages, and marketing claims unsupported by data, Labdoor offers something rare: lab-verified clarity. Its certification is not a guarantee of benefit—but it is a strong signal that a supplement contains what it says it does, and avoids what it shouldn’t. For consumers who want to make informed decisions based on measurable facts, rather than advertising hype, Labdoor provides a critical foundation.

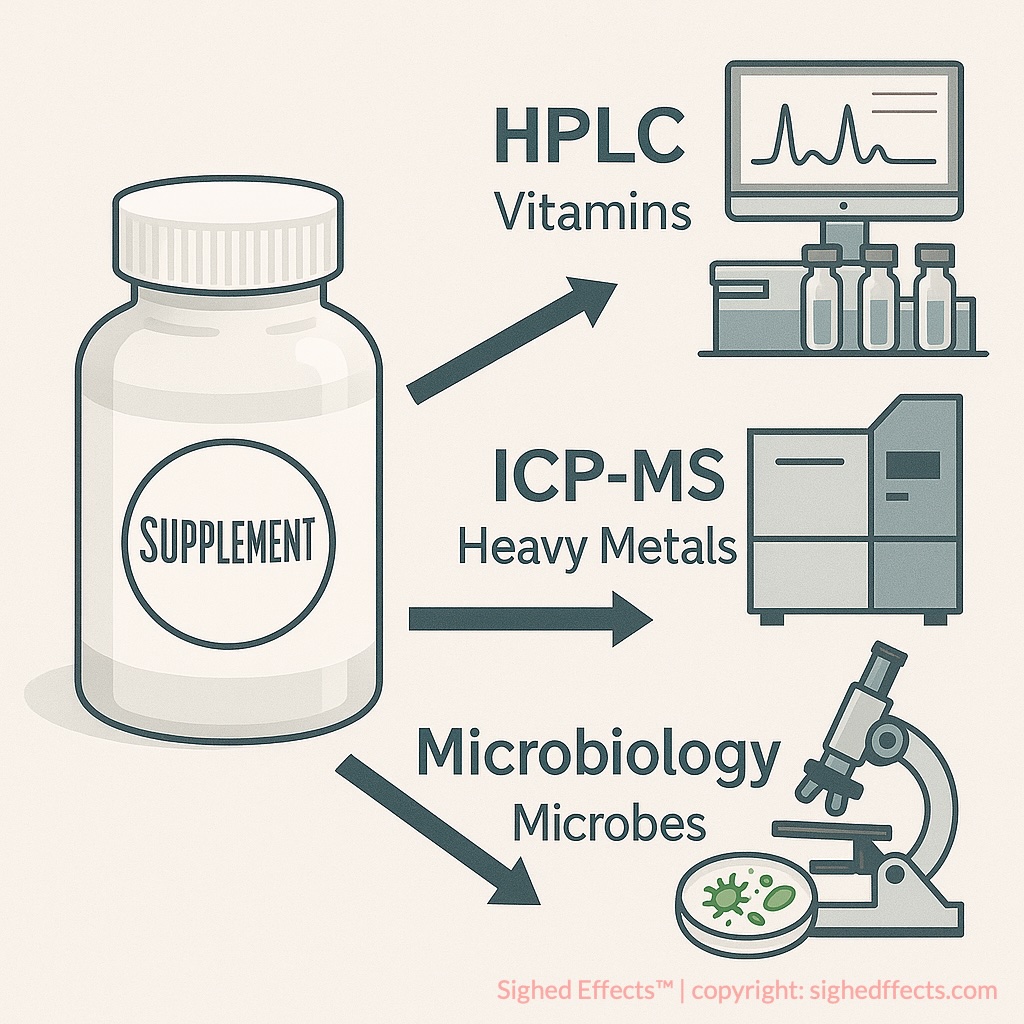
How Labdoor Tests Supplements
Labdoor’s supplement testing process is built on analytical chemistry, standardized protocols, and third-party laboratory partnerships. The company sends unopened supplement products—usually purchased anonymously from retail shelves or online stores—to FDA-registered and ISO 17025-accredited laboratories. These labs perform a series of chemical analyses designed to verify label claims, detect contaminants, and assess overall product safety.
Testing the Finished Product
Unlike manufacturing audits or supply chain certifications, Labdoor focuses solely on the finished product that a consumer would receive. This is critical in a largely self-regulated industry where front-facing branding often obscures quality control issues, underdosing, or undeclared substances. Labdoor’s goal is simple: test what’s actually inside the bottle, not just what the label says.
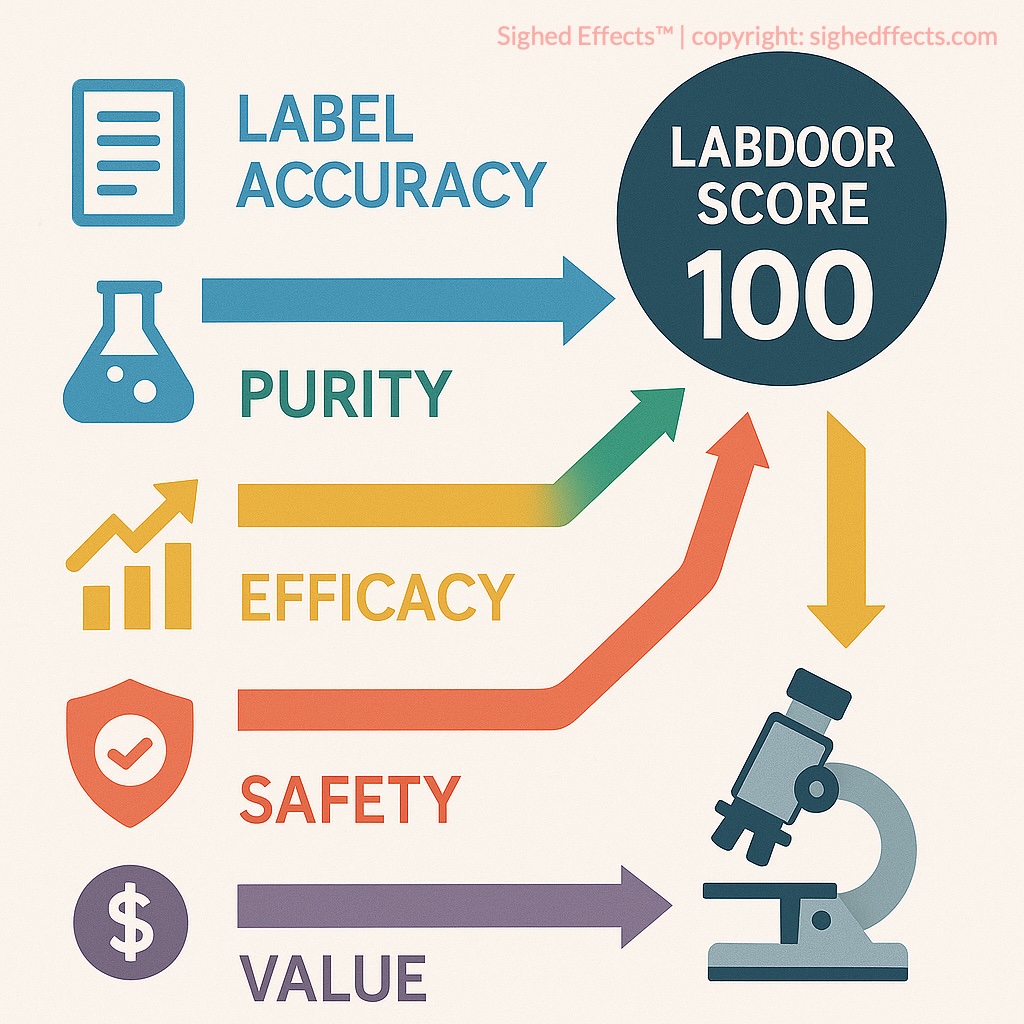
Labdoor Scoring Pillars
The core of Labdoor’s methodology revolves around three pillars: label accuracy, purity, and value. Each supplement is assigned a weighted score across five dimensions:
- Label Accuracy: Do the active ingredients match what’s listed on the label?
- Product Purity: Are there any contaminants like heavy metals, microbes, or unlisted substances?
- Nutritional Value: How much beneficial content does the product deliver per unit cost?
- Ingredient Safety: Are the ingredient dosages within safe, clinically acceptable ranges?
- Projected Efficacy: Do the ingredients match evidence-based dosing guidelines?
Analytical Methods Used
Labdoor relies on gold-standard analytical tools commonly used in pharmaceutical testing. These include:
- High-Performance Liquid Chromatography (HPLC): to separate, identify, and quantify compounds such as vitamins, amino acids, and plant actives.
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): to detect trace levels of heavy metals like arsenic, cadmium, lead, and mercury.
- Gas Chromatography–Mass Spectrometry (GC-MS): in certain cases to test for banned substances or residual solvents.
- Microbiological assays: to screen for microbial contamination such as E. coli, yeast, mold, and salmonella.
These tests are conducted under standardized conditions using validated methods, which ensures reproducibility across batches. Labdoor does not develop its own lab protocols; instead, it follows compendial testing methods such as USP or AOAC standards to maintain scientific rigor.
Scoring and Public Reporting
Once the lab results are compiled, Labdoor’s in-house team analyzes the data and translates it into a category-specific score. The final “Labdoor Score” is reported on a scale from 0 to 100 and integrates the weighted factors above. A score above 90 typically reflects a product that meets or exceeds label claims, shows no signs of contamination, and delivers clinically meaningful ingredient levels. Lower scores may indicate mislabeling, dosage inconsistencies, or elevated risk.
Examples of Labdoor Findings
Consider a protein powder that claims 25 grams of protein per serving. If testing reveals it contains only 19 grams and elevated levels of lead, it will be penalized for both label inaccuracy and safety concerns. These deductions are clearly noted in the product’s public report.
Another example involves multivitamins. Labdoor has tested dozens of formulations and often finds significant variability in nutrient dosing—some delivering 300% of the RDA for a vitamin, others failing to meet even 50% of the claimed amount. Products that fall outside accepted thresholds are marked down accordingly, with transparency in the scoring rationale.
What’s Included in Labdoor Reports
Once a product is fully evaluated, Labdoor publishes the results in a public-facing report. This includes:
- Full lab results for each tested ingredient
- Detected contaminants with reference safety levels
- Comparison to label claims
- Details on testing date and methodology
- Ranking position within its supplement category
This level of public access sets Labdoor apart from most other certification programs, which may issue a seal but keep underlying data private. With Labdoor, the data drives the badge—not the other way around.
How Labdoor Is Used by Consumers and Professionals
Consumers, clinicians, and even regulators have used Labdoor’s reports to cross-verify supplement quality, especially when working with patients who rely on consistent dosing. This is particularly relevant for nutrients like magnesium, vitamin D, creatine, or fish oil, where clinical outcomes are dose-dependent and deviations can impact efficacy or safety.
Labdoor’s Impact
In summary, Labdoor’s testing approach is not just about detecting fraud—it’s about validating integrity. By focusing on the end product and publishing results openly, Labdoor helps consumers cut through noise, marketing, and misinformation with verifiable facts.
What the Labdoor Score Actually Means
The Labdoor Score is a numerical rating from 0 to 100 that reflects the scientific integrity, label transparency, and overall safety of a dietary supplement. But unlike a star rating or crowd-sourced review, the Labdoor Score is not subjective. It is data-driven, based on third-party laboratory analysis of ingredient content, contamination risks, and dose relevance. Understanding what this score represents—and what it does not—is key to using it as a reliable benchmark in a crowded and unregulated marketplace.
How the Score Is Calculated
At its core, the Labdoor Score is a composite metric built from several individually weighted categories. Each product tested is evaluated across five dimensions, each of which contributes to the final rating:
- Label Accuracy (30%): The percentage by which the actual content of each active ingredient matches what is printed on the label.
- Product Purity (25%): Measurement of harmful substances such as heavy metals, microbes, or undeclared compounds.
- Nutritional Value (20%): Assessment of ingredient concentration in relation to price, absorption potential, and clinical relevance.
- Ingredient Safety (15%): Analysis of dosage safety relative to established tolerable upper intake levels (ULs) and toxicological data.
- Projected Efficacy (10%): An estimate of whether the product is dosed in a way that aligns with evidence-based clinical outcomes.
The weights of each dimension are publicly listed, and Labdoor periodically updates them as scientific standards evolve. For example, if new data emerges showing that bioavailability varies significantly between two forms of an ingredient (such as magnesium oxide vs. magnesium glycinate), Labdoor may adjust how efficacy is factored into the score for that product type.
What the Score Does and Doesn’t Mean
Importantly, the Labdoor Score does not reflect personal results, taste, brand reputation, or customer satisfaction. It is not a measure of how well a product “works” for any individual, but rather a rigorous comparison of what is actually in the bottle versus what is claimed, and whether that aligns with clinical expectations and safety limits.
Examples of Score Application
Consider two examples: a vitamin D3 supplement that claims 5,000 IU per serving but delivers only 3,200 IU may score poorly for label accuracy. A protein powder contaminated with cadmium, even if it meets protein content claims, will be penalized heavily under the purity and safety metrics. A multivitamin dosed well below clinically relevant thresholds—even if it’s free of contaminants—might receive a low efficacy score. This scoring system helps consumers go beyond advertising claims and into the chemical reality of each product.
Category Normalization
Labdoor also normalizes scores within categories, so that products with vastly different intended uses can’t be unfairly compared. For instance, omega-3 fish oil supplements are ranked against other fish oil supplements—not against creatine powders or collagen blends. This ensures that each score reflects performance within a relevant peer group.
What High and Low Scores Indicate
When a supplement receives a score of 90 or above, it generally means the product has:
- Accurately dosed active ingredients (within ±10% of the label)
- Minimal to no detectable contamination with heavy metals or microbes
- Ingredient forms that are supported by clinical research
- No ingredients that exceed known safety thresholds
Scores below 70 often reflect significant issues—such as large dosing discrepancies, use of poorly supported proprietary blends, or detection of potentially harmful substances. In some cases, these findings have led consumers to avoid certain products entirely or prompted brands to reformulate in response to public transparency.
Score Breakdown and User Priorities
One of the most helpful aspects of the Labdoor Score is its detailed breakdown. Each scorecard includes both the overall rating and the individual dimension scores, allowing users to decide which factors matter most to them. For example, a healthcare provider may prioritize purity, while an athlete may focus more on label accuracy and efficacy.
Labdoor Score as a Comparison Tool
Additionally, the score provides a useful filter when browsing by category. Rather than relying on brand marketing or influencer endorsements, consumers can sort supplements by a transparent, test-based standard. Labdoor’s ranking interface allows users to compare products head-to-head, complete with price per dose and purity metrics.
Clinical Use of Labdoor Scores
For clinicians, the Labdoor Score offers a starting point for therapeutic decision-making. While it does not replace clinical judgment or individual health screening, it can confirm whether a supplement is delivering what it claims—and avoid products that may introduce avoidable risk through contaminants or underdosing.
Score Updates and Relevance
Lastly, the Labdoor Score is dynamic. Scores may be updated when new batches are tested, methodologies evolve, or ingredient standards change. This means users should always check the date of the most recent evaluation and view the score as a current snapshot—not a permanent verdict.
Summary: Decoding the Label
In essence, the Labdoor Score is a precision instrument for decoding the supplement label. It rewards honesty, penalizes risk, and gives consumers an evidence-based lens through which to evaluate their options. Whether you’re a casual buyer or a health professional, it adds a measurable layer of certainty in an industry that often lacks it.
How Labdoor Differs from USP, NSF, and Informed Choice
Labdoor is often grouped with third-party supplement certifiers like USP (U.S. Pharmacopeia), NSF International, and Informed Choice—but the similarities are more superficial than functional. Each of these organizations operates under a distinct model with different priorities, and understanding these differences is essential for interpreting what their certifications mean on a product label.
Product Selection and Testing Process
The most fundamental difference lies in how Labdoor selects and tests products. Labdoor is unique in that it independently purchases supplements from retail outlets or online stores—just as a regular consumer would. The products are then anonymously submitted for testing. This eliminates brand influence in the selection process and ensures that the exact product being evaluated is representative of what users actually receive. In contrast, USP and NSF certifications typically begin with the manufacturer submitting a product sample and initiating a paid agreement for testing, auditing, or licensing.
Manufacturing vs. Finished Product Testing
Most traditional certification bodies also emphasize manufacturing practices over finished product testing. USP and NSF, for example, include facility audits, documentation reviews, and adherence to GMP (Good Manufacturing Practice) protocols. These processes assess whether the brand follows proper procedures during production but do not always confirm what is inside the final product that reaches consumers. Informed Choice, which is more focused on athletic supplements, does include banned substance testing on finished batches—but again, only for participating brands.
Labdoor’s Consumer-Focused Model
Labdoor reverses this model: it starts with the product itself, testing only the finished supplement—exactly as it appears on store shelves. This gives it a unique role in the market, functioning more like a consumer watchdog than a compliance consultant. In this way, Labdoor’s certification badge reflects verified label accuracy, actual contaminant levels, and measured ingredient quantities, not just supply chain integrity or GMP alignment.
Transparency and Data Access
Another defining characteristic of Labdoor is its open data policy. Every product tested by Labdoor receives a full, public report that includes:
- Quantified ingredient content (e.g., 492 mg of vitamin C vs. a 500 mg claim)
- Contaminant testing results with reference limits (e.g., lead below Prop 65 thresholds)
- Ranking methodology and numerical breakdown by scoring dimension
- Date of testing and lab methods used
In contrast, many third-party certifications provide minimal public data. A USP Verified or NSF Certified badge typically confirms that a product passed certain audits or met label standards at the time of submission, but no detailed test results or scoring breakdowns are shared. Consumers must trust that the certifier’s internal standards were met—without the ability to examine the data themselves.
Scope of Supplement Categories
Labdoor also differs in category breadth. While NSF Certified for Sport and Informed Choice are designed specifically for athletes concerned about banned substances and doping compliance, Labdoor focuses on a much broader set of supplements, including:
- General-use vitamins and minerals
- Protein powders and meal replacements
- Omega-3 fish oil and krill oil
- CBD tinctures and softgels
- Creatine and performance enhancers
- Probiotics, magnesium, and electrolytes
This makes Labdoor a more accessible tool for the average consumer, whereas NSF and Informed Choice are primarily used in sports, clinical, or professional settings. USP tends to focus on more traditional supplement categories, such as single-nutrient vitamins and minerals, and does not actively test newer or trending formulations.
Business Model and Independence
Another important distinction is business model transparency. Labdoor publishes products even if the brand did not ask to be tested. Some manufacturers discover they’ve been tested only after the report is published. They cannot suppress, delay, or negotiate the score. If their product performs poorly, the results go live anyway. This is not the case with most pay-to-participate models, which test only enrolled products and often require licensing fees for the certification mark to be used in marketing.
Purpose and Use Cases
Of course, this does not mean Labdoor is “better” than other certifiers—it means it serves a different purpose. USP and NSF provide rigorous GMP oversight and manufacturing quality assurance. Informed Choice helps protect athletes from anti-doping violations by batch-testing for prohibited substances. These services are essential in contexts where regulatory compliance, insurance coverage, or international trade requirements apply.
When to Use Labdoor
Labdoor, by contrast, is built for consumer-facing quality verification. Its greatest strength is that it measures what’s delivered—not just what’s intended. It is especially useful for:
- Identifying underdosed or mislabeled supplements
- Flagging hidden contaminants in over-the-counter products
- Comparing products side by side within a supplement category
- Empowering users who want data over branding or anecdote
In essence, Labdoor differs from USP, NSF, and Informed Choice in mission, method, and message. Where others validate procedures, Labdoor validates outcomes. Where others certify supply chains, Labdoor certifies contents. And while others focus on compliance frameworks, Labdoor focuses on scientific transparency for everyday users.
Summary: Labdoor’s Distinct Role
For consumers and practitioners seeking a clearer picture of supplement integrity based on real lab data, Labdoor offers a uniquely actionable layer of protection. It doesn’t replace the need for clinical expertise or manufacturing audits—but it does illuminate what’s in the bottle when it counts most.
Is Labdoor Certification Reliable?
Reliability in the supplement world means more than good intentions. It requires scientific rigor, transparent methodology, independent validation, and reproducibility. Labdoor Certification meets these standards by offering consumers a consistently structured, publicly documented, and third-party verified system for analyzing dietary supplements. But like any certification, its reliability depends on how well users understand what it can—and cannot—guarantee.
Accredited Lab Partnerships
First and foremost, Labdoor works with FDA-registered and ISO 17025-accredited laboratories. These labs are held to the highest international standards for analytical accuracy and procedural integrity. ISO 17025 accreditation specifically requires labs to demonstrate technical competence, validation of methods, regular calibration of equipment, and full documentation of results. It is the same accreditation used for clinical and pharmaceutical labs.
Testing Methods and Rigor
The lab methods employed include High-Performance Liquid Chromatography (HPLC), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), and microbiological assays. These tools allow precise quantification of active ingredients, detection of trace contaminants, and screening for microbial pathogens. Labdoor does not rely on simple assay kits or visual inspection—it uses pharmaceutical-grade testing techniques to assess supplement content at the molecular level.
Transparency and Public Data
A key strength of Labdoor is its transparency-first model. All testing data is published on the Labdoor platform and is accessible to anyone, free of charge. Each supplement’s report includes a list of ingredients tested, their measured quantities, the margin of error from label claims, and any detected contaminants. It also provides the testing date, lab methods used, and how each sub-score was calculated. This level of public disclosure is unusual in the supplement industry, where many certification results are proprietary.
Examples of Transparency
For example, if a protein powder claims 25 grams of protein per serving but delivers only 20 grams, the exact discrepancy is published. If a fish oil supplement contains trace mercury just below regulatory thresholds, that level is included in the product report. Nothing is concealed. Brands cannot pay to alter or remove findings.
Manufacturer Independence
Another factor enhancing Labdoor’s reliability is its independence from manufacturers. Unlike certification bodies that depend on brand enrollment or licensing revenue to stay operational, Labdoor often tests products without brand consent. Products are purchased anonymously and submitted for analysis without notifying the manufacturer. While companies can later license the Labdoor badge for marketing use, the evaluation process itself occurs independently, before any commercial relationship exists.
This approach reduces the potential for bias, selective reporting, or sample manipulation. It also avoids the “cherry-picking” problem, where brands might send ideal production runs for third-party testing while releasing lower-quality batches to the public. With Labdoor, the tested product is the consumer product.
What Labdoor Does Not Cover
However, reliability does not mean comprehensiveness. Labdoor is highly reliable within the scope it covers—but that scope is limited. It does not conduct audits of manufacturing facilities, verify batch traceability, or review GMP compliance documentation. It also does not evaluate brand ethics, labor sourcing, or sustainability metrics. For those dimensions, other certification systems (e.g., NSF GMP or Fair Trade Certified) may be more appropriate.
Labdoor also cannot guarantee consistency across all batches. It tests a single lot or sample at a time. If a product changes formula or sourcing after being tested—or if there is high variability across production runs—those issues might not be captured until retesting occurs. This is why checking the “Last Tested” date is important when using Labdoor reports. A supplement tested in 2019 may no longer reflect its 2025 formulation.
Real-World Impact
Still, Labdoor’s reliability is bolstered by its methodology and intent: it exists to protect the consumer by providing scientifically grounded, independent verification of what a supplement actually contains. For many supplement categories—especially those with known quality control problems, like fish oil, magnesium, or protein powders—Labdoor’s evaluations have revealed discrepancies that would otherwise go unnoticed.
Influence on Industry and Healthcare
For instance, Labdoor’s testing has identified products with less than 60% of claimed active ingredients, protein spiking with cheap nitrogen sources, and the presence of banned stimulants not disclosed on product labels. These findings have triggered recalls, FDA warnings, and widespread consumer pushback—demonstrating real-world impact.
The healthcare sector has also taken notice. While not an official regulatory body, Labdoor has been cited in publications by physicians, registered dietitians, and public health researchers as a valuable tool for verifying supplement quality. Many practitioners use Labdoor’s data to screen products before recommending them to patients, especially when clinical outcomes depend on precise nutrient dosing.
Limitations of the Scoring System
In terms of limitations, Labdoor’s scoring system is sometimes criticized for assuming equivalence between forms (e.g., magnesium oxide vs. glycinate), though recent updates have improved this by factoring in bioavailability more directly. Likewise, not all ingredients are tested equally—botanical extracts with highly variable chemistry may receive less definitive results than single-molecule compounds like creatine monohydrate or ascorbic acid.
Context for Reliability
Despite these limitations, the reliability of Labdoor Certification holds strong when interpreted within context. It offers a powerful layer of quality control in an industry where regulatory oversight is often minimal. It is not a guarantee of clinical efficacy or ethical manufacturing, but it is a validated check against mislabeling, contamination, and underperformance.
Summary: Using Labdoor for Assurance
For consumers seeking data, not just promises, Labdoor Certification provides a critical filter. For professionals seeking reassurance about product quality, it provides a verified starting point. And for an industry too often built on opacity, Labdoor delivers one of the few tools designed to put science—and accountability—on the label.

Which Types of Supplements Does Labdoor Test?
Labdoor selects supplements for testing based on public interest, clinical relevance, and risk of misrepresentation. Its portfolio spans widely used health products as well as those known to carry risks of label inaccuracy or contamination. While it does not claim to test every supplement on the market, Labdoor’s coverage includes some of the most commonly consumed—and commonly misrepresented—product types.
The following categories are among Labdoor’s most frequently tested:
- Multivitamins: These are tested for the full panel of nutrients, including vitamin A, B-complex, C, D, E, and minerals such as calcium, magnesium, iron, and zinc. Labdoor checks for under- and overdosing, inappropriate forms (e.g., cyanocobalamin vs. methylcobalamin), and undeclared additives like artificial colorants or sweeteners.
- Protein Powders: These are screened for protein concentration, amino acid profile, and contamination with heavy metals such as lead, arsenic, and cadmium. Labdoor also checks for “nitrogen spiking”—a deceptive practice where companies artificially inflate protein content by adding cheap nitrogen-rich compounds like creatine or taurine.
- Omega-3 Fish Oil: Supplements in this category are tested for EPA and DHA content, oxidation levels (via peroxide and anisidine values), and contamination from mercury or polychlorinated biphenyls (PCBs). Labdoor has previously flagged products that failed purity thresholds due to rancidity or environmental pollutants.
- Creatine Monohydrate: Creatine is a widely used performance-enhancing supplement. Labdoor evaluates it for purity, solubility, and label accuracy. Products are also screened for contaminants such as dicyandiamide or heavy metals introduced during synthesis.
- CBD Oils and Capsules: These are tested for cannabinoid content, THC levels, residual solvents from extraction, and heavy metal contamination. Labdoor often finds significant variance in actual vs. claimed cannabidiol (CBD) concentrations, with some products delivering less than 50% of the labeled dose.
- Probiotics: These supplements are tested for total colony-forming units (CFUs) of declared strains, viability over shelf life, and presence of harmful microbes. Labdoor also reviews whether products actually contain the strains claimed on the label, which is particularly important for clinical use.
- Magnesium and Electrolyte Supplements: Products are tested for elemental magnesium content, contaminant levels, and consistency between servings. Due to widespread label inaccuracy in this category, Labdoor prioritizes magnesium oxide, citrate, glycinate, and malate formulations for purity and dosage verification.
- Vitamin D, B12, and C: These single-ingredient products are tested for potency and stability, since degradation over time is a common issue. Water-soluble vitamins like B12 and C are especially prone to breakdown under heat or light exposure, and Labdoor checks for potency loss compared to label claims.
- Pre-Workout Supplements: Labdoor screens these for caffeine content, stimulant consistency, and potential inclusion of banned or undeclared substances. Proprietary blends are flagged for lack of dosing transparency, and ingredients like beta-alanine and L-citrulline are measured against effective dose ranges.
Labdoor also considers the risk profile and formulation complexity of a category before choosing to test it. Products with a high likelihood of mislabeling—such as proprietary blends, multi-ingredient stacks, or imported herbal extracts—are prioritized if sufficient consumer demand or safety concern exists.
In contrast, supplements that are difficult to standardize for chemical analysis (e.g., complex botanical extracts, adaptogen blends, or homeopathic formulas) may be excluded unless a validated testing method is available. This reflects a scientific limitation, not a lack of importance.
Testing coverage also evolves over time. For instance, as consumer demand for CBD surged in the early 2020s, Labdoor added cannabidiol oils and capsules to its testing roster—despite regulatory ambiguity and widespread market fraud. Similarly, categories like collagen, nootropics, and greens powders may be added as methodologies improve and market size justifies scrutiny.
It’s important to note that Labdoor typically publishes results for the top-selling or most widely used products within each category. This means the platform does not test every brand, but it does provide comparative data across those that dominate market share. This offers a practical entry point for consumers making buying decisions.
Additionally, Labdoor allows consumers to request product testing. A form on the Labdoor website enables users to nominate supplements for analysis. If a product receives significant interest or if it has a history of quality concerns, it may be prioritized for future evaluation.
In summary, Labdoor focuses its testing efforts on supplements that are:
- Widely consumed or clinically relevant
- Known to have label discrepancies or purity issues
- Quantifiable using validated chemical testing methods
- Sold directly to consumers without tight regulatory oversight
While it does not aim to be exhaustive, Labdoor’s selection of supplement types provides a highly relevant snapshot of the products most likely to be used—and misused—by the general public. Its testing portfolio reflects not just market size, but also public health significance.

Limitations of Labdoor Certification
Labdoor Certification provides consumers with an important tool to assess supplement quality, but it is not a comprehensive guarantee of safety, efficacy, or ethical manufacturing. Understanding the boundaries of Labdoor’s methodology is critical for using its ratings appropriately. Like any certification system, it has inherent limitations that stem from its scope, resources, and mission.
First and foremost, Labdoor does not evaluate manufacturing processes or facilities. Unlike NSF or USP, which conduct on-site GMP audits, Labdoor does not visit factories, inspect supply chain records, or validate the origin of raw ingredients. It tests only the finished product. This means a supplement might pass Labdoor’s purity and dosage tests yet still be produced in a facility with poor hygiene, inadequate quality control, or labor violations. Labdoor explicitly focuses on the end-user experience, not upstream compliance.
Another limitation is the absence of **batch consistency testing**. Labdoor typically analyzes only one lot or batch of each product—the version available at the time of retail purchase. If a manufacturer introduces formulation changes, experiences batch contamination, or inconsistently fills capsules, these variations may go undetected unless multiple retests are performed. This introduces a risk of assuming consistent quality based on a single snapshot.
To mitigate this, Labdoor publishes the testing date and allows consumers to track whether a product has been retested. However, not all products are retested regularly. For supplements with high variability, such as those containing live probiotics or botanical extracts, batch-to-batch differences can be significant—and potentially clinically relevant.
Labdoor also does not conduct clinical efficacy studies. The “Projected Efficacy” score is based on a comparison between the product’s dosage and published clinical research on that ingredient. This provides a useful estimate—but it does not measure real-world absorption, user outcomes, or bioindividual effects. For example, a magnesium supplement that contains 200 mg per serving may be scored as clinically relevant, but Labdoor does not assess whether that dose is absorbed efficiently or effective in a specific health context.
Similarly, Labdoor does not test for drug–nutrient interactions or contraindications. A supplement may contain levels of vitamin K, calcium, or herbal extracts that could interact with common medications or medical conditions. Labdoor reports do not flag these risks, and users must seek medical guidance before using any supplement—especially if they are taking prescription drugs or managing chronic illness.
There is also limited scope in testing complex or poorly defined ingredients. Many supplements contain proprietary blends that include plant extracts, adaptogens, or nootropic compounds. While Labdoor can measure individual nutrients like creatine or EPA, it is often more difficult to quantify or verify ingredients like ashwagandha extract, mushroom blends, or bioactive peptides—especially when their active compounds are not well standardized across the industry.
In cases where standard chemical analysis is not feasible, Labdoor may choose not to test certain ingredients at all, or may report the overall blend weight without verifying individual components. This makes it difficult for consumers to assess the therapeutic relevance of such formulations, even if the supplement performs well in purity or safety categories.
Ethical, environmental, and supply chain considerations are also outside Labdoor’s scope. The certification does not evaluate whether a supplement is vegan, organic, sustainably sourced, fair trade, or cruelty-free. Nor does it address carbon emissions, packaging waste, or labor practices. While these factors are important to many consumers, they fall under the domain of other certifiers such as USDA Organic, Fair for Life, or Certified Vegan.
Additionally, Labdoor does not perform **longitudinal safety tracking**. If a supplement causes adverse effects after extended use or in specific populations (e.g., pregnant individuals, children, athletes), Labdoor’s testing process will not capture those risks. Nor does it operate a pharmacovigilance system or maintain adverse event databases. Its certification is based on the chemical content of the product, not its physiological impact over time.
There is also a visibility limitation. Labdoor has tested hundreds of supplements—but thousands exist. Many products remain untested simply due to scale and resource constraints. While consumers can request testing for new products, and Labdoor may prioritize popular or high-risk categories, there is no guarantee that a given supplement will be evaluated in a timely manner.
Despite these limitations, Labdoor’s value lies in its narrow focus: testing what’s in the bottle and disclosing it to the public. Within that scope, it operates with a level of transparency and scientific rigor that few other organizations offer. But it should not be mistaken for a seal of clinical approval, manufacturing audit, or long-term safety validation.
To use Labdoor effectively, consumers and professionals should treat it as one lens among many. It is best combined with:
- Clinical consultation or medical advice
- Review of GMP certifications and supply chain documentation
- Consideration of sustainability, allergens, and ethical sourcing
- Ongoing monitoring of product changes and formulation updates
In short, Labdoor does not replace critical thinking—it supports it. It does not verify everything, but it verifies what most certifications don’t: that a product contains what it claims to, and that it’s free of dangerous contaminants. For informed supplement decisions, that’s a powerful place to start.

How to Use Labdoor Rankings as a Consumer or Clinician
Labdoor rankings are designed to simplify complex lab data into a usable format—but knowing how to apply them in real-world decision-making is key. Whether you’re a consumer navigating hundreds of product options or a clinician guiding patient supplement use, understanding the strengths and limits of the ranking system can help transform raw numbers into informed choices.
The most direct way to use Labdoor is to compare products within a given category. Each ranking page (e.g., for creatine, multivitamins, or fish oil) lists supplements by their overall Labdoor Score, with numerical breakdowns for label accuracy, purity, nutritional value, ingredient safety, and projected efficacy. This allows users to see not only which products perform well, but why.
For consumers, this can simplify decisions in otherwise opaque categories. A shopper comparing five brands of magnesium glycinate can immediately identify which options deliver the stated dosage, avoid heavy metal contamination, and meet safe intake levels. They can also filter results by price per serving or dosage efficiency—an important feature in a market where some high-priced supplements offer little more than low-cost competitors.
For clinicians, Labdoor offers a layer of assurance that the product being recommended meets basic quality benchmarks. This is especially relevant for:
- Patients with chronic conditions who rely on precise dosing (e.g., iron for anemia, vitamin D for bone health)
- Patients with limited liver or kidney function who may be vulnerable to trace contaminants
- Pregnant or breastfeeding individuals where contaminant thresholds and ingredient purity are critical
- Gastrointestinal or absorption issues where form and dose matter greatly (e.g., magnesium oxide vs. citrate)
Labdoor can also serve as a tiebreaker when two brands seem comparable in formulation. For instance, if two creatine supplements both list “creatine monohydrate” as the sole ingredient, Labdoor testing might reveal that one contains a higher percentage of active compound or is free of impurities linked to lower-quality synthesis processes.
However, it’s important not to over-interpret the numerical score. The difference between a Labdoor Score of 91 and 88 may be based on small variations in dosing accuracy or pricing—not necessarily safety concerns. Consumers should always review the sub-scores and read the product report to understand where deductions occurred. In some cases, a slightly lower-ranked product may still be preferable due to better tolerance, availability, or formulation (e.g., capsules vs. powders).
Another tip for using Labdoor is to verify whether the product tested is the same version currently sold. Labdoor includes the date of testing and often includes batch identifiers. If a supplement has been reformulated since testing—especially in terms of dosage, added ingredients, or manufacturing source—the ranking may no longer apply. Clinicians should double-check label updates and contact manufacturers directly if necessary.
Labdoor is also helpful for detecting underperforming products that are still widely sold. If a supplement ranks poorly due to inaccurate dosing, unsafe contaminant levels, or use of ineffective ingredient forms, that is usually reflected in a significantly lower score. In categories like omega-3 fish oil or protein powders—where mislabeling and oxidation are common—this can help users avoid otherwise popular products that don’t meet minimum quality standards.
In terms of workflow integration, clinicians may consider creating shortlists of top-ranked products within frequently recommended categories. For example:
- Top 3 multivitamins with accurate dosing and no heavy metals
- Top 2 vegan omega-3 products with verified EPA/DHA content
- Best creatine powders with minimal price per gram and high purity
These shortlists can be integrated into EMRs, patient handouts, or supplement protocols, helping standardize recommendations and improve treatment consistency.
Consumers may also use Labdoor proactively during purchasing. Before buying a product online or in-store, they can search the name on Labdoor’s site to see if it’s been tested. If the product hasn’t, users may choose an alternative that has been independently verified—or submit a request for Labdoor to evaluate it.
Labdoor rankings also play a role in filtering through influencer marketing noise. Many supplements are promoted on social media using anecdotal evidence, aesthetic appeal, or brand loyalty—rather than testing or clinical relevance. A quick Labdoor search can help confirm whether the product matches its hype. If it hasn’t been tested, or ranks poorly despite popularity, that signals a need for caution.
Additionally, users can explore the ranking reports by score dimension. For example, someone primarily concerned with ingredient safety can sort products by that metric, even if overall scores are similar. A product may perform poorly in pricing efficiency but excel in purity—a tradeoff some users may accept.
In summary, Labdoor rankings are best used as a starting point for supplement selection—not a final verdict. They help confirm whether a product delivers on label claims, avoids common contaminants, and is dosed in line with evidence-based expectations. But final decisions should always consider:
- Current formulation and testing date
- Patient-specific needs or medical context
- Budget, access, and supplement delivery form
- Clinical relevance of ingredients included
For both consumers and clinicians, Labdoor offers a unique tool: a window into the chemical reality behind supplement marketing. It can’t replace good judgment—but it can sharpen it.

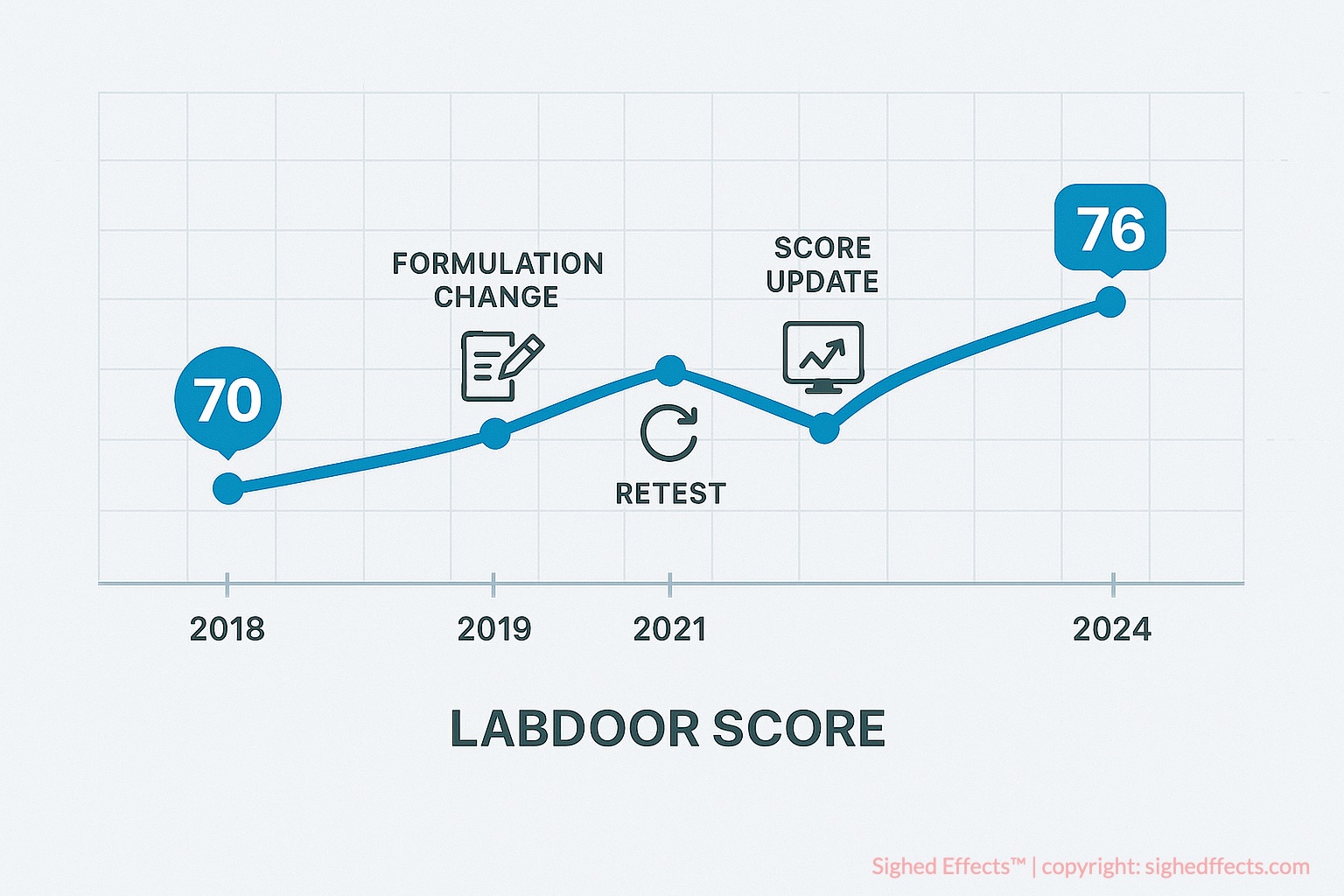
Can a Labdoor Score Change Over Time?
Yes, a Labdoor Score is not fixed permanently—it can change over time based on new testing, reformulations, manufacturing adjustments, or shifts in Labdoor’s evaluation criteria. This dynamic aspect reflects the real-world variability in supplement production and is one of the reasons Labdoor remains a relevant and living resource for supplement evaluation.
Labdoor periodically retests products, especially those that are popular, reformulated, or flagged for past concerns. If a manufacturer changes a product’s ingredient profile, sourcing, dosage, or manufacturing process, Labdoor may acquire the new batch and submit it for fresh analysis. These new results can lead to a revised score and updated ranking within the supplement category.
For example, in 2021, Labdoor retested a popular fish oil supplement that previously scored 88 due to accurate EPA/DHA content and low oxidation markers. However, the newer batch showed elevated peroxide values, indicating potential rancidity. The product’s score was adjusted downward to 73, and Labdoor flagged the report with a notice explaining the change. This case illustrates the importance of batch-based transparency—what was once a top-tier product may decline in quality if oversight lapses or supplier standards shift.
The process of retesting is integral to maintaining up-to-date supplement rankings. Supplement formulations are not static—manufacturers may switch ingredient suppliers, alter dosages, or introduce new excipients, sometimes in response to cost pressures or changes in regulatory requirements. These modifications can have a direct impact on the quality, safety, and efficacy of the finished product. By periodically purchasing and testing new batches from retail channels, Labdoor captures these changes and ensures that its scores reflect the current market reality, not just historical performance.
Scores may also change when Labdoor updates its internal scoring algorithms or weighting systems. For example, if new scientific consensus emerges around a contaminant threshold or clinical dosage range, Labdoor may adjust how much weight is assigned to those factors in the overall Labdoor Score. This ensures that the rankings reflect current standards of safety and efficacy, rather than outdated assumptions.
In 2023, Labdoor modified its evaluation criteria for magnesium supplements to account for differences in bioavailability across forms (e.g., magnesium oxide vs. glycinate). As a result, several products with previously high scores saw minor reductions—not due to contamination or label inaccuracy, but because projected efficacy was reweighted in favor of forms with higher clinical absorption. This adaptive scoring reflects a commitment to keeping the rating system aligned with the latest scientific evidence.
When Labdoor revises its scoring model, it often does so in consultation with scientific literature, clinical guidelines, and feedback from both consumers and industry experts. For instance, if new research demonstrates that a certain contaminant poses a greater health risk than previously thought, Labdoor may increase the penalty for its presence in tested products. Similarly, if clinical studies reveal that a specific ingredient form is far more bioavailable than alternatives, the projected efficacy weighting may be recalibrated. These algorithmic updates are designed to ensure that Labdoor’s rankings are not only accurate, but also relevant to current best practices in nutrition and toxicology.
In some cases, scores decrease because a product’s new batch performs worse than its original version. This may happen due to supply chain issues, cost-cutting measures, or poor quality control. Conversely, a brand may improve its standing by increasing ingredient accuracy, reducing contamination, or eliminating unnecessary fillers—resulting in a higher score upon retesting.
The impact of these score changes can be significant for both consumers and manufacturers. For consumers, it underscores the importance of consulting the most recent Labdoor report before making a purchase, rather than relying on outdated rankings or word-of-mouth reputation. For manufacturers, it serves as a strong incentive to maintain consistent quality across all batches and to respond proactively to any flagged issues. A drop in Labdoor Score can lead to lost sales, negative publicity, and increased scrutiny, while an improved score can boost consumer confidence and brand credibility.
Labdoor’s platform clearly indicates when a product has been retested and includes dates for when testing was performed. This allows users to determine whether the information is current and relevant. However, not all products are retested frequently, and some reports may remain static for months or even years, especially if the product is discontinued or if consumer demand for retesting is low.
Consumers can also submit retesting requests through Labdoor’s public platform, which allows user input to influence prioritization. When multiple users raise concerns or report issues with a product, Labdoor may prioritize that product for batch verification. This crowdsourced feedback loop gives consumers a participatory role in maintaining supplement accountability.
For the most accurate use of Labdoor scores, users should always check the “Last Tested” date listed in each product report. When in doubt, it may be worth contacting the manufacturer directly to confirm whether a product has been reformulated since the time of testing.
Ultimately, the evolving nature of the Labdoor Score reflects the evolving nature of the supplement industry itself. It is a reminder that quality is not a one-time achievement—but an ongoing commitment. The supplement market is characterized by rapid innovation, shifting regulations, and global supply chains, all of which introduce variability into product quality. Labdoor’s approach—combining regular retesting, algorithmic updates, and transparent reporting—provides a mechanism for holding brands accountable and for keeping consumers informed as the landscape changes.
In summary, a Labdoor Score is not a static stamp of approval, but a living indicator of a product’s current standing in the marketplace. It can rise or fall based on new test results, scientific advances, or changes in manufacturing practices. By paying attention to retesting dates, algorithm changes, and flagged notices, consumers can make more informed choices and avoid the pitfalls of outdated or misleading supplement ratings. Labdoor’s commitment to ongoing evaluation ensures that its scores remain a trustworthy resource in an industry where quality can shift rapidly and unpredictably.
Is Labdoor Certification Enough on Its Own?
Labdoor Certification offers consumers a rare layer of clarity in the supplement market—but is it sufficient on its own to determine whether a product is truly worth taking? The answer depends on context. While Labdoor excels at verifying what’s inside a supplement bottle, it does not offer a complete picture of every risk, benefit, or ethical concern associated with supplement use. In this final section, we’ll explore where Labdoor fits—and where its boundaries require additional tools or frameworks.
Labdoor’s core strength is chemical verification. It confirms whether the ingredients in a supplement match the label, whether they are present in safe quantities, and whether the product is contaminated with harmful substances like heavy metals, microbes, or unlisted compounds. In a largely unregulated market, this is no small feat. However, supplement quality is not the same as supplement suitability. A product can be pure, accurately dosed, and still be ineffective—or even harmful—for a specific user.
For example, a multivitamin with high doses of vitamin A and iron may be well-formulated from a technical standpoint, but it may not be appropriate for individuals with hemochromatosis, liver conditions, or pregnancy without supervision. Labdoor does not assess drug–nutrient interactions, genetic vulnerabilities, or personal medical history. It cannot determine whether a supplement is right for you—it can only determine whether it is honest and uncontaminated.
Similarly, Labdoor does not verify health claims, therapeutic outcomes, or performance benefits. If a product is marketed to improve cognition, reduce inflammation, or enhance athletic recovery, Labdoor does not evaluate whether those claims are supported by human clinical trials. The “Projected Efficacy” score is based solely on ingredient dosing relative to published literature—it does not measure how well the supplement actually works in the body.
This makes Labdoor an excellent filtering tool, but not a complete endorsement engine. Consumers should still evaluate:
- Whether the supplement’s ingredients are evidence-based for the intended use
- Whether they are appropriate for personal medical history or current medications
- Whether the delivery format, timing, and co-factors are optimized for absorption
- Whether long-term use is advisable or clinically justified
In this sense, Labdoor should be viewed as a quality control gate, not a prescription or recommendation. It can flag poor-quality products, verify safe ones, and elevate the credibility of a brand—but it cannot replace medical guidance or tailored protocols. Its results are best used in tandem with:
- Healthcare provider input (especially for vulnerable populations)
- Third-party research tools like Examine.com or PubMed
- Ingredient-specific databases or meta-analyses
- Complementary certifications for ethical or allergen concerns
For example, someone purchasing a fish oil supplement may want to combine Labdoor verification with:
- IFOS or GOED certification for oxidation and sustainability standards
- ConsumerLab or USP data for comparison across different sample lots
- Marine Stewardship Council (MSC) verification for eco-sourcing
Or consider a consumer buying a turmeric supplement for inflammation. Labdoor can verify curcumin content and absence of heavy metals—but it cannot assess whether the product includes necessary co-factors like piperine for absorption, or whether the dose aligns with trials used in rheumatoid arthritis. That analysis requires clinical interpretation.
Additionally, Labdoor does not address consumer-specific values such as:
- Vegan or allergen-free formulation
- Religious dietary standards (e.g., kosher, halal)
- Non-GMO or organic certification
- Fair trade or ethical labor sourcing
These factors often influence purchasing decisions just as much as chemical quality. While Labdoor helps ensure the product is not dangerous or deceptive, it does not inform whether it is aligned with the consumer’s broader ethical or health framework.
That said, the simplicity of Labdoor’s certification can be an asset. For individuals new to supplements, overwhelmed by choice, or skeptical of marketing claims, the Labdoor Score offers a data-driven anchor point. It provides immediate insight into whether a product is fundamentally sound, free of red flags, and worthy of further consideration. In a marketplace where many products are poorly regulated, that foundation is both rare and powerful.
For advanced users—practitioners, researchers, athletes, or health-savvy consumers—Labdoor serves as a gatekeeper for quality assurance. Once a product passes that gate, the user can apply additional layers of analysis: clinical matching, pharmacokinetics, cofactor compatibility, or value per serving.
In summary: Labdoor Certification is necessary for identifying high-integrity supplements, but it is not always sufficient for making a final decision. It should be used as part of a broader strategy that includes evidence-based dosing, ingredient synergy, patient history, and ethical considerations. In this way, Labdoor becomes not a replacement for judgment—but a powerful tool that improves it.
This article is part of our Certifications hub — Our deep dives into third-party testing, purity standards, and label verification systems across the supplement industry..
Questions or Comments?
If you have a question or comment about this article, feel free to leave it below. All comments are moderated for clarity, accuracy, and relevance.

2 Comments
How often does Labdoor update their rankings? If a supplement improves its formula, how quickly would that be reflected in their score?
Labdoor updates their rankings periodically, but not in real time. Products are only re-evaluated when Labdoor conducts a new round of testing or when a brand pays for a retest, which Labdoor discloses on the product’s profile page.
If a supplement improves its formula, the change will not be reflected immediately in its Labdoor score. The update depends on:
• Whether Labdoor independently selects the product for new testing in its next cycle.
• Or whether the manufacturer requests and pays for a retest, which includes submitting the updated formula and allowing Labdoor to purchase the product from a retail source for verification.
Even with a retest, Labdoor maintains strict controls: the product must be bought independently (not supplied by the company), and the new formulation must pass all testing metrics before the score is adjusted. So while updates are possible, there may be weeks or even months of lag time between a formula change and its reflection on Labdoor’s platform.